For U.S. Residents Only

An Important Announcement From 
RENASSIST will not reopen in 2018, but will remain open through December 21, 2017.
See Important DatesIndication
Renvela® (sevelamer carbonate) is used to control phosphorus levels in adults and children 6 years of age and older with chronic kidney disease (CKD) on dialysis.
Important Safety Information
Do not use Renvela if you have a history of bowel obstruction or if you are allergic to sevelamer carbonate, sevelamer hydrochloride, or to any of the ingredients in Renvela.
Click here for additional Important Safety Information.
Please see Full Prescribing Information for Renvela (PDF).
Please see Full Prescribing Information for Sevelamer Carbonate (PDF).
Sevelamer carbonate—the authorized generic identical to Renvela®
Winthrop US is a Sanofi company dedicated to delivering brand-equal generics of Sanofi products, including Renvela.
The truth behind generics
What is a generic drug? A generic drug is a copy of a brand-name drug that is made by a company other than the company that makes the brand-name. A generic drug is the same as the brand-name drug in terms of active ingredient, conditions of use, dosage form, strength, route of administration, and (with certain permissible differences) labeling. However, a generic drug may have certain minor differences from the brand-name product, such as different inactive ingredients or tablet size.
In contrast, an authorized generic drug is the exact same in all aspects as a brand-name drug, except that it is marketed without the brand name on its label.
Choosing Winthrop US sevelamer carbonate ensures that you will get the exact same drug product as branded Renvela.
Winthrop US's sevelamer carbonate uses the same manufacturing process as Renvela. It is identical to Renvela in shape, active ingredients, size, and inactive ingredients.
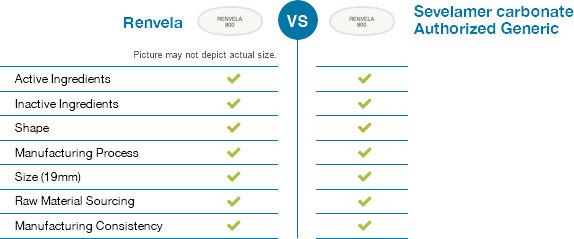
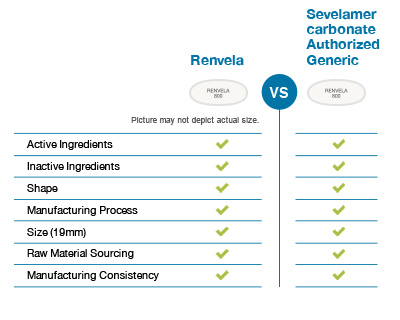
Affordable solutions
From the earliest stages of development to batch release, Winthrop US is dedicated to providing affordable solutions to the healthcare community. Sevelamer carbonate comes with all the quality of Renvela along with the cost savings of generics.
Learn more about Winthrop US
See more details about the authorized generic sevelamer carbonate, as well as the other products Winthrop US has to offer.

Renvela®
Indication
Renvela® (sevelamer carbonate) is used to control phosphorus levels in adults and children 6 years of age and older with chronic kidney disease (CKD) on dialysis.
Important Safety Information
- Do not use Renvela if you have a history of bowel obstruction or if you are allergic to sevelamer carbonate, sevelamer hydrochloride, or to any of the ingredients in Renvela.
- Talk to your doctor if you have had difficulty swallowing or swallowing disorders; or if you have had digestive tract surgery or other digestive disorders, including severe constipation.
- The most common side effects with sevelamer include vomiting, nausea, diarrhea, indigestion, abdominal pain, flatulence, and constipation.
- Cases of hypersensitivity, itching, rash, stomach pain, bleeding gastrointestinal ulcers, colitis, ulceration, necrosis, fecal impaction and less commonly, slow bowel activity, bowel obstruction, and bowel perforation have been reported.
- Cases of difficulty swallowing the Renvela tablet have been reported. Talk to your doctor if you have difficulty swallowing medicines in tablet form. Renvela powder for oral suspension may be considered by your doctor if you have a history of difficulty swallowing.
- Your doctor should monitor bicarbonate and chloride blood levels.
- Reduced vitamins D, E, and K (clotting factors) and folic acid blood levels may be followed by your doctor.
- Talk to your doctor when taking sevelamer with other medications.
- Promptly contact your doctor if you experience severe abdominal pain, new or worsening constipation, or other severe intestinal symptoms while on sevelamer.
- Take sevelamer with meals and adhere to your prescribed diet
Sevelamer Carbonate
Indication
Sevelamer carbonate is used to control phosphorus levels in adults and children 6 years of age and older with chronic kidney disease (CKD) on dialysis.
Important Safety Information
- Do not use sevelamer carbonate if you have a history of bowel obstruction or if you are allergic to sevelamer carbonate, sevelamer hydrochloride or to any of the ingredients.
- Talk to your doctor if you have had difficulty swallowing or swallowing disorders; or if you have had digestive tract surgery or other digestive disorders, including severe constipation.
- The most common side effects with sevelamer include vomiting, nausea, diarrhea, indigestion, abdominal pain, flatulence, and constipation.
- Cases of itching, rash, fecal impaction and, less commonly, slow bowel activity, bowel obstruction, bleeding gastrointestinal ulcers, colitis, ulceration, necrosis and bowel perforation have been reported.
- Uncommon cases of difficulty swallowing the sevelamer carbonate tablet have been reported. Talk to your doctor if you have difficulty swallowing medicines in tablet form. Sevelamer carbonate for oral suspension may be considered by your doctor if you have a history of difficulty swallowing.
- Your doctor should monitor your bicarbonate and chloride blood levels.
- Reduced vitamins D, E, and K (clotting factors) and folic acid blood levels may be followed by your doctor.
- Talk to your doctor when taking sevelamer carbonate with other medications.
- Promptly contact your doctor if you experience severe abdominal pain, new or worsening constipation, or other severe intestinal symptoms while on sevelamer carbonate.
- Take sevelamer carbonate with meals and adhere to your prescribed diet.
Please see Full Prescribing Information for Renvela (PDF).
Please see Full Prescribing Information for Sevelamer Carbonate (PDF).
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.




 Team
Team